Cochrane workshop «New tools for developing and assessing the quality of evidence-based clinical guidelines for safety of pharmacotherapy», organized by Cochrane Russia together with Cochrane China, was held as a part of the Second Russian Congress «Safety of pharmacotherapy 360°: Noli Nocere!» at RMANPO on May 23, 2024.
Seminar recording
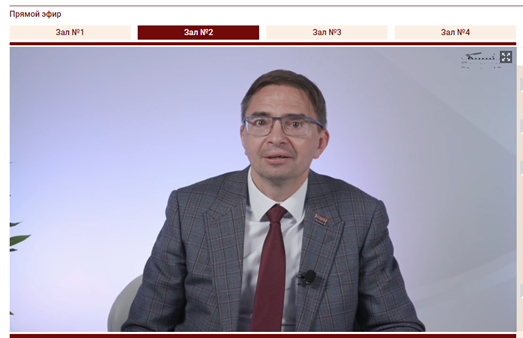
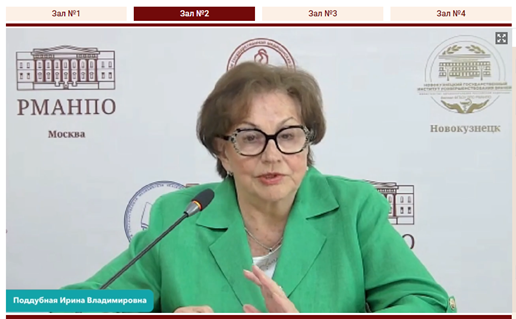
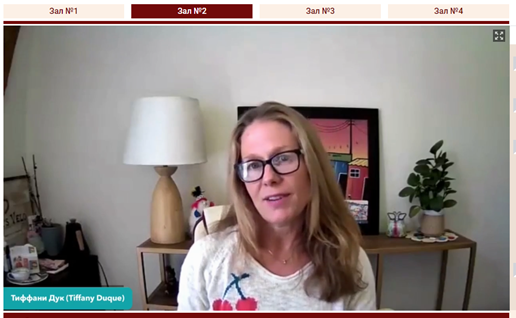
The Rector of RMANPO, Academician of the Russian Academy of Sciences (RAS) Dmitry A. Sychev, the Vice-Rector of RMANPO, Academician of RAS Irina V. Poddubnaya, Senior Officer of US Network & Geographic Groups and Cochrane Central Executive Team Tiffany Duque and Cochrane Russia Director Prof. L. E. Ziganshina addressed the participants of the workshop with welcoming speeches.
They wished all participants and speakers a successful and fruitful workshop and noted the success of Cochrane Russia work at RMANPO in the capacity of the Knowledge Translation Centre. The main streams of work, that were emphasized by the leaders were the development of collaboration with Cochrane China. Inclusion of Cochrane workshops and evidence-based medicine topics in scientific and educational events of RMANPO became a valuable tradition. The topic of this workshop was described as particularly relevant due to the growing need for high-quality clinical guidelines as the main information tool for the provision of medical care.
 |  |
 |  |
The event was moderated by the Rector, Dmitry A. Sychev, the Vice-Rector Irina V. Poddubnaya and Director of Cochrane Russia, Professor Liliya Eu. Ziganshina.
Janne Estill, Associate Professor at the School of Basic Medical Sciences of Lanzhou University [10:20], together with Professor Yaolong Chen, presented the lecture “RIGHT tool for developers and reviewers of clinical practice guidelines in healthcare. Perspectives for the development of the RIGHT methodology”, in which he spoke about the RIGHT tool, the RIGHT checklist, the development of checklists for reporting information on medical research, as well as extended versions of RIGHT, their testing and updating.
 | 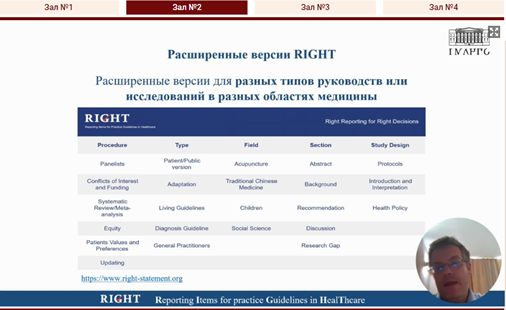 |
Professor Chavdar S. Pavlov [45:15] in his presentation focused on the ethical aspects of the development of clinical guidelines, conflicts of interest, issues of the quality of reporting evidence and overestimation of the strength of evidence in clinical guidelines, the reasons of inconsistency between results of Cochrane reviews and clinical guidelines’ recommendations.
 | 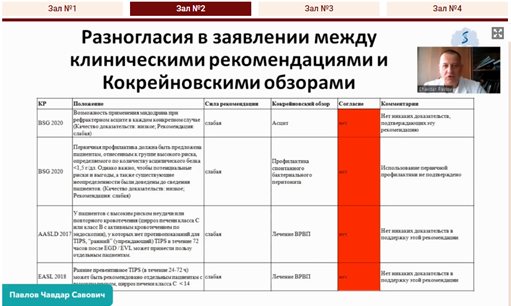 |
Ekaterina V. Yudina [1:05:37] introduced the workshop participants to the Russian-language version of RIGHT, published in the Kazan Medical Journal and presented on the official RIGHT website and noted the importance of the RIGHT tool for the target audience.
 |  |
Mikhail S. Chenkurov [1:10:38] presented on behalf of the Department of General and Clinical Pharmacology of the Peoples' Friendship University of Russia, named after Patrice Lumumba. In his talk he presented the results of the pioneering study on assessement of the quality of Russian clinical practice guidelines on the use of oral anticoagulants for prevention of thrombotic complications in patients with coronavirus infection using AGREE and RIGHT tools.
 | 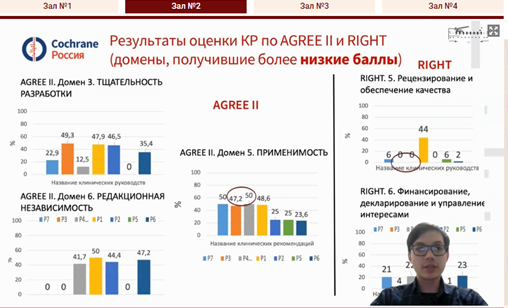 |
In the final report, Professor Liliya Eugenevna Ziganshina [1:23:00] spoke about Cochrane Russia contribution to universal access to Cochrane evidence, the 10-years of the Russian translation project of Cochrane evidence, the national subscription to the Cochrane Library and the main achievements of Cochrane Russia and the Cochrane Collaboration.
 | 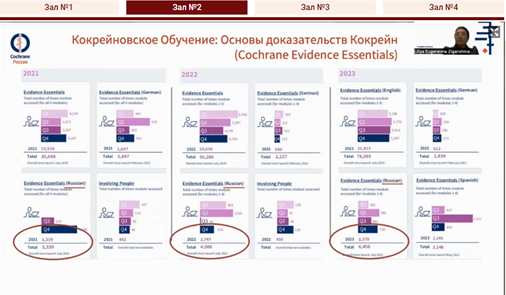 |
Concluding the workshop, the Vice-Rector of RMANPO Irina V. Poddubnaya summed up the results of the event, noted the large number of participants and expressed hope for the continuation of the tradition of including Cochrane workshops in scientific and educational activities of RMANPO and the active participation of academy staff in the use of Cochrane evidence and Cochrane activities.
We thank all the participants of the workshop! See you at our future events!
